With current or previous funding from The Michael J. Fox Foundation, several drugs are now in clinical trials, with volunteers testing the potential of these drugs to slow or stop Parkinson’s disease progression or to ease symptoms.
Our Approach
None of these therapies would be possible without the support of generous donors and research participants. Here we outline where MJFF-backed medications are in the drug development pipeline and what they aim to do.
To find recruiting studies in your area:
There are five stages of development for new therapies, ranging from small-scale testing to long-term evaluation after regulatory approval.
Disease-modifying
These therapies aim to prevent, slow or halt the overall progression of Parkinson's disease (PD). They target different proteins and pathways believed to play a role in the disease.
-
Alpha-synuclein
This protein forms toxic clumps in some brain and body cells of people with PD.
-
Anle138b
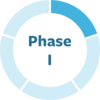
MODAG's small molecule aims to inhibit aggregation of alpha-synuclein. MJFF funded pre-clinical work and a portion of a Phase I trial in people with Parkinson's.
-
BIIB054
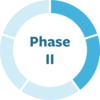
Biogen’s antibody aims to prevent aggregated alpha-synuclein from spreading. MJFF is funding tool development and data collection that support study design.
-
NPT088
Proclara’s (previously Neurophage) drug candidate aims to prevent alpha-synuclein from clumping together. MJFF funded pre-clinical work.

-
PD01A
AFFiRiS’ vaccine aims to stimulate antibodies against alpha-synuclein. MJFF funded pre-clinical work, a portion of the Phase I trial and boost studies.

-
RO7046015
Prothena/Roche’s antibody aims to prevent aggregated alpha-synuclein from spreading. MJFF is funding tool development and data collection that support study design.

-
-
GBA
Mutations in the GBA gene are associated with Parkinson’s disease and are linked to certain cellular dysfunction.
-
GZ/SAR402671
Sanofi Genzyme’s drug reduces production of lipids that build up with GBA mutations. MJFF is funding tool development and data collection that support study design.

-
LTI-291
Lysosomal Therapeutics’ oral drug may offset dysfunction associated with GBA mutation. MJFF funded the pre-clinical work.

-
-
LRRK2
Mutations in the LRRK2 gene are associated with Parkinson’s disease and linked to greater activity of the LRRK2 protein.
-
DNL201
Denali’s LRRK2 inhibitor aims to lower heightened LRRK2 activity. MJFF funded safety studies that supported the trial.

-
-
Repurposed Drugs
Some drugs approved for other conditions could be beneficial for people with PD.
-
Exenatide
Diabetes medication that has protected brain cells in pre-clinical Parkinson’s models. MJFF funded the Phase II trial led by the University of College London.

-
Inosine
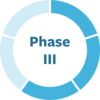
Nutritional supplement raises urate (antioxidant) levels. Population studies show inosine may have a protective effect or slow progression of PD. MJFF funded pre-clinical work and the Phase II trial and is supporting biomarker collection in the Phase III trial led by the Parkinson Study Group.
-
Isradipine
High blood pressure drug may help protect brain cells. MJFF funded the pre-clinical work and the Phase II trial and is supporting biomarker collection in the Phase III trial led by the Parkinson Study Group.

-
Nilotinib
This treatment for a cancer of the white blood cells (chronic myelogenous leukemia) may address dysfunction seen in PD. MJFF is funding a Phase II trial led by the Parkinson Study Group.

-
-
Neurotrophic Factors
Trophic factors are like the brain's natural fertilizer; they help restore and protect neurons.
-
GDNF
MedGenesis’ trophic factor may protect dopamine cells. MJFF funded the pre-clinical work.

-
CDNF
Herantis’ trophic factor may protect dopamine cells. MJFF funded the pre-clinical work.

-
Motor Symptoms
Tremor, stiffness and slowness of movement affect mobility. Levodopa can help, but it does not treat all symptoms, can feel less effective with time and may bring side effects such as dyskinesia with long-term use.
-
Levodopa Delivery
The gold standard for motor symptom treatments can, with long-term use, wear off and cause side effects, such as dyskinesia. Researchers believe some side effects may be due to fluctuating levels of levodopa.
-
Accordion Pill
Layers of levodopa/carbidopa release slowly from the stomach for better absorption. MJFF funded the pre-clinical work by Intec Pharma.

-
NDO612
Neuroderm’s levodopa/carbidopa pump or pump-patch could maintain steady levels of levodopa. MJFF funded a portion of Phase I and Phase II trials.

-
-
Non-dopamine Approaches
Targeting other brain chemicals with add-on therapies may help control motor fluctuations associated with levodopa use.
-
PXT002331
Prexton Therapeutics’ oral drug (foliglurax) works on the glutamate and other brain chemical systems to reduce motor symptoms and dyskinesia. MJFF funded the pre-clinical work.

-
-
"Off" Rescue
When levodopa levels diminish, patients’ symptoms can return; this is called an "off" episode.
-
APL-130277
Sunovion’s (previously Cynapsus) thin film of the drug apomorphine placed under the tongue could rescue patients from "off" episodes. MJFF funded Phase I and Phase II trials.

-
CVT-301
Acorda’s (previously Civitas) inhaled levodopa can quickly ease symptoms. MJFF funded Phase I and Phase II trials.

-
-
Gene Therapy
With surgery, a selected gene is delivered to the brain to increase production of deficient protein.
-
AAV2-hAADC
Voyager’s approach aims to replace the enzyme AADC in brain cells to improve levodopa conversion to dopamine for better control of motor symptoms and less “off” time in advanced Parkinson’s. MJFF funded a Phase I trial.

-
-
Dyskinesia
A potential side effect of long-term levodopa use, dyskinesia is involuntary, sometimes jerky, movement.
-
ADX48621-201
Addex Pharma’s oral drug (dipraglurant) blocks the effects of the brain chemical glutamate, which may decrease dyskinesia. MJFF funded the pre-clinical work and a Phase II trial.

-

-
-
Gait and Balance
Gait instability and falls are often resistant to or only partially responsive to current therapies that work to increase the dopamine lacking in Parkinson's.
-
Donepezil
Drug acts on brain chemical acetylcholine and has been shown to reduce falls in PD patients. MJFF funded a Phase II trial by Oregon Health & Sciences University. (Donepezil is also in testing for Parkinson’s cognition.)

-
Varenicline
Medication targets the brain chemical acetylcholine, which may be related to imbalance and falls. MJFF is funding a Phase II trial at University of Michigan.

-
Non-motor Symptoms
Researchers are increasingly recognizing the impact of non-motor symptoms on quality of life.
-
Anxiety
Mood changes such as anxiety and depression are symptoms of Parkinson's.
-
Buspirone
Drug is used to treat some forms of anxiety. MJFF is funding a Phase II trial at the University of Rochester.

-
-
Constipation
Decreased bowel movements can lead to discomfort and erratic levodopa absorption.
-
Resistant Starch
Nutritional supplement may improve gut bacteria and ease constipation. MJFF is funding a Phase II trial at Saarland University.

-
RQ-10
Novel drug that works on the serotonin chemical system may speed stomach emptying and lessen constipation. MJFF is funding a Phase I trial at Virginia Commonwealth University.

-

Be Part of the Answer
You have the power to impact your future and the future of millions living with Parkinson's disease. Explore clinical research participation today.
