The LRRK2 Investigative Therapeutics Exchange (LITE) is a comprehensive, large-scale global collaboration designed to fast-track therapies that target LRRK2 and identify and optimize reliable biomarkers that can guide treatments. LITE brings together academic researchers, industry experts and clinicians to share data, tools and discoveries in real time.
LRRK2 is a gene that helps to regulate how cells function and maintain their health. It also happens to be one of the most promising and intensively studied genetic targets in Parkinson’s.
When changes or mutations happen in LRRK2, the protein can become overactive and disrupt normal cellular processes. These mutations are one of the most known causes of inherited Parkinson’s disease. However, increasing evidence suggests that an overactive LRRK2 pathway may contribute to Parkinson’s even in people without inherited mutations. Researchers and companies are highly active in developing therapies to reduce excess LRRK2 activity, as these treatments could help people with LRRK2 mutations and potentially many others with Parkinson’s that involves the same biological pathway.
Innovation through Collaboration
In traditional research and drug development, academic labs, private companies and clinical practitioners work independently, protecting their data and discoveries until publication or patent. The LITE program works differently. It connects more than 50 partners from across these sectors who agree from the start to share data, biosamples, tools and early findings. This creates an environment where collaborators work together on promising ideas and move them from hypothesis to practical therapy faster. With access to shared resources, discoveries can advance from early-stage research into clinical development more efficiently and with less risk.
Conference Proceedings on LRRK2
In June 2025, The Michael J. Fox Foundation hosted a workshop that brought together researchers, clinicians, industry representatives and funders to identify collaborative strategies and research priorities to accelerate clinical development of LRRK2-targeted therapies for Parkinson’s disease. The full report, "Advancing Development of LRRK2-Targeted Therapeutics for Parkinson’s Disease: Conference Proceedings and Roadmap for Research," is published by RAND and available for download.
LITE is designed to accelerate progress toward new therapies through a global consortium built on the principles of early collaboration, open communication and long-term commitment.
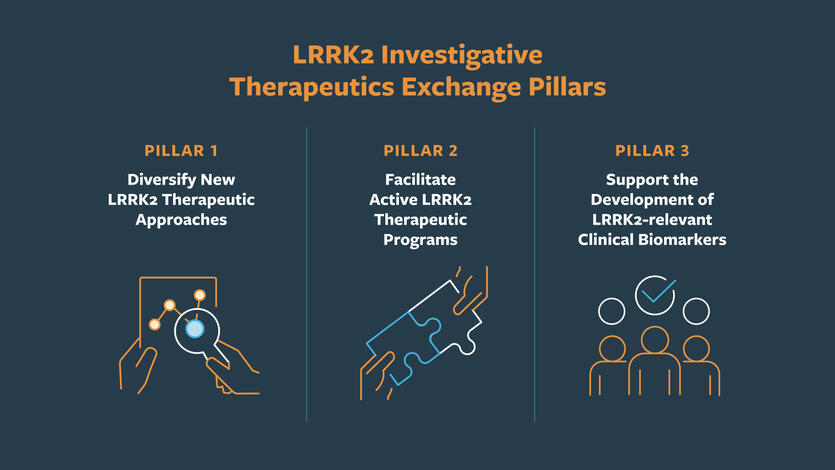
The three pillars of the program are based on using collaborative science to address key gaps in LRRK2 translational research.
-
Pillar 1: Diversify New LRRK2 Therapeutic Approaches
Discover and test innovative strategies to modulate the LRRK2 pathway. In drug development, the more biological approaches we explore, the more likely we are to find success. LITE is continually expanding therapeutic approaches to LRRK2, with an array of programs including small molecules, biologics, and chemical probes. Researchers within LITE also share compounds and screening platforms, enabling faster evaluation and comparison of potential therapeutic mechanisms.
-
Pillar 2: Facilitate Active LRRK2 Therapeutic Programs
Support academic and industry teams as they advance LRRK2-targeted therapies from discovery into preclinical and clinical development. This includes access to model systems, assay platforms, medicinal chemistry expertise, and opportunities for collaboration with experienced drug developers. Unified and streamlined support from LITE reduces time, cost, and risk for individual teams.
-
Pillar 3: Support the Development of LRRK2-relevant Clinical Biomarkers
Identify and validate biomarkers that can detect LRRK2-related activity, monitor disease progression, and help select the right participants for clinical trials. For success in clinical trials, we need both promising therapeutic approaches and tools to measure therapeutic effects of those interventions. Efforts include testing molecular biomarkers, imaging tools, and digital health measures. Current studies are evaluating these markers in people with and without LRRK2 mutations to understand who is most likely to benefit from future therapies.
The goal of LITE is to reduce risk and uncertainty in developing LRRK2 therapies, helping promising programs move from early discovery into clinical testing faster and more confidently.
To make this possible, LITE is building shared infrastructure, including global cohorts of people with and without Parkinson’s, standardized laboratory tools and open-access data platforms that allow researchers to test LRRK2 biomarkers and therapeutic strategies efficiently and consistently. By creating this collective framework, LITE lowers costs, avoids duplication of effort and enables treatments to reach the clinic sooner than would be possible through isolated projects.
Three-Year Goals
- By the end of this three-year program, LITE aims to:
- Test more than two dozen therapeutic approaches to target LRRK2 and identify promising molecules that could become future therapies or shared tools for the research community.
- Build and actively share preclinical and clinical tools, including establishing a new global cohort of individuals with LRRK2 mutations contributing biosamples and clinical data, now in progress across multiple global sites.
- Begin testing candidate biomarkers to learn whether they can identify individuals most likely to benefit from LRRK2 therapies, which can help design smarter clinical trials.
- Create assay platforms, cell models, and chemical tools that can be openly shared and applied to targets beyond LRRK2, so this collaborative approach can accelerate more therapeutic areas in the future.
Open Science
LITE operates on a structured open-science framework that requires active data and resource sharing across all participating institutions. Academic groups, biotech companies and pharmaceutical partners contribute and access shared datasets, biospecimens, validated assays and experimental results rather than working in separate, siloed pipelines.
This collaborative model accelerates therapeutic development by reducing redundancy, enabling cross-validation, and ensuring that findings are reproducible across independent sites. It also supports efficient decision-making, which allows for promising discoveries to advance and for unsuccessful approaches to be identified earlier.
Leadership
The initiative is governed by a steering committee consisting of staff from The Michael J. Fox Foundation and key advisors with drug discovery and LRRK2 biology expertise. It is implemented by the University of Dundee in the United Kingdom with strong collaborations from more than 20 academic key opinion leaders, more than 20 biotech and pharma key opinion leaders and more than 10 clinical advisors.
LITE is led by Dario Alessi, PhD, who runs a lab focused on kinase research at the University of Dundee in the United Kingdom. Dr. Alessi’s colleague Esther Sammler, MD, PhD, serves as co-principal investigator on the program. University of Dundee UK’s Paul Davies, PhD, and Francesca Tonelli, PhD, join them as part of the study’s leadership. The study also receives key guidance from advisors Stevan Djuric, PhD; Alastair Reith, PhD; and Darryle Schoepp, PhD.
Each pillar of the program also receives direction from leaders in the field. The first pillar, Diversity New LRRK2 Therapeutic Approaches, is co-led by Kalpana M. Merchant, PhD, of TransThera Consulting Co. and Northwestern University Feinberg School of Medicine. Pillar 2, Facilitate Active LRRK2 Therapeutic Programs, is co-led by Prof. Mahmood Ahmed of University of Dundee. Prof. Christine Klein, MD, FEAN, of University of Luebeck, advises the third pillar, Support the Development of LRRK2-relevant Clinical Biomarkers, with program management support from Eva-Juliane Vollstedt, MD, of University of Luebeck and University Hospital Schleswig-Holstein, Campus Luebeck.
Collaborative Initiatives
A related project focusing on LRRK2 signaling pathways is part of the Aligning Science Across Parkinson’s (ASAP) Collaborative Research Network (CRN), creating a point of collaboration across initiatives. The LITE program also benefits from collaboration with other ASAP initiative-supported programs including the Parkinson’s Progression Markers Initiatives (PPMI) and the Global Parkinson’s Genetics Program (GP2).
Academic Collaborators
-
Cincinnati Children's Hospital Medical Center
Dr. Jeffrey Whittset, Dr. William Zaccharias, Dr. Jichao Chen
-
National University of Singapore
Dr. Ji Sun
-
St Jude Children’s Research Hospital
Dr. Christoph Gorgulla
-
University of Toronto
Dr. Aled Edwards, Dr. Levon Halabelian
-
Stanford University
Dr. Monther Abu-Remaileh, Dr. Nathaniel Gray
-
Stanford University School of Medicine
Dr. Suzanne Pfeffer
-
Trinity College Dublin
Dr. Amir Khan
-
University of Dundee
Dr. Mahmood Ahmed, Dr. Alessio Ciulli, Dr. Will Farnaby, Dr. Peter Cossar, Dr. Prasad Jalagam
-
University of Frankfurt
Dr. Stefan Knapp
-
University of North Carolina
Dr. Alison Axtman, Dr. Rafael Counago
-
University of San Diego
Dr. Andres Leschziner, Dr. Sam Reck Peterson
-
University of San Francisco
Dr. Kevan Shokat
-
VIB
Dr. Wim Versees
-
Calibr-Skaggs Institute for Innovative Medicine
Dr. Michael Bollong, Dr. Kristen Johnson
Industry Collaborators
Key to the success of LITE is the participation of industry partners, who leverage the resources made available through the initiative as well as share back information and tools to benefit the broader Exchange.


Contact Us
Do you represent a company that is working on LRRK2 therapeutics and want to know more about how your organization can contribute to and benefit from the program? Please contact grants@michaeljfox.org.


