Many physical symptoms of Parkinson’s disease (PD) are easy to see — motor fluctuations, difficulty walking, tremors. However, when it comes to understanding what happens inside the body to make those symptoms occur, the “seeing” part becomes much more complicated.
What we need is an imaging test to allow us to see how Parkinson’s happens inside the brain, much like the way radiological scans can reveal how cancer spread throughout the body. The work is ongoing, but obstacles remain. First, we need tracers that can serve as beacons for signs of Parkinson’s in the brain. Second, we need imaging systems that can zero in on the smallest, hardest to reach parts of the brain.
“Imaging makes up an important part of our biomarker strategy,” says Jamie Eberling, PhD, senior vice president of research resources at MJFF. “It has the potential to speed clinical trials by showing how important parts of Parkinson’s biology function in the brain, including the regions most affected and changes over time.”
The Hunt for Parkinson’s Tracers
Tracers are radioactive substances used during a Positron Emission Tomography (PET) scan that can detect disease and evaluate how well an organ is functioning. For example, there are tracers that can cause cancer cells to “light up” during a scan. But we do not yet have tracers that detect the best-known aspects of PD biology, like accumulation of the protein alpha-synuclein, which clumps up in the brains of people with Parkinson’s. An alpha-synuclein tracer would allow us to see when the protein starts to accumulate and track its progression as the disease advances. This would be a critical step for early diagnosis as well as an important contributor to faster, more effective clinical trials, since progress could be measured over time (and we could tell if a treatment slowed progression).
Tracers remain elusive, but we are making progress. For the first time, researchers presented more than one alpha-synuclein PET tracer in clinical trials at the 2025 AD/PD conference. Merck Pharmaceuticals and Massachusetts General Hospital both have tracers in development (with funding from MJFF), as does Dr. Makoto Higuchi, from the Advanced Neuroimaging Center, Institute for Quantum Medical Science, all based at the National Institutes for Quantum Science and Technology (QST) in Japan. These tracers have shortcomings, and none of them are fully validated (vetted scientifically) by the field. But the field is moving closer to a tracer that could be used widely for clinical trials and impacts patient care.
Improving Imaging
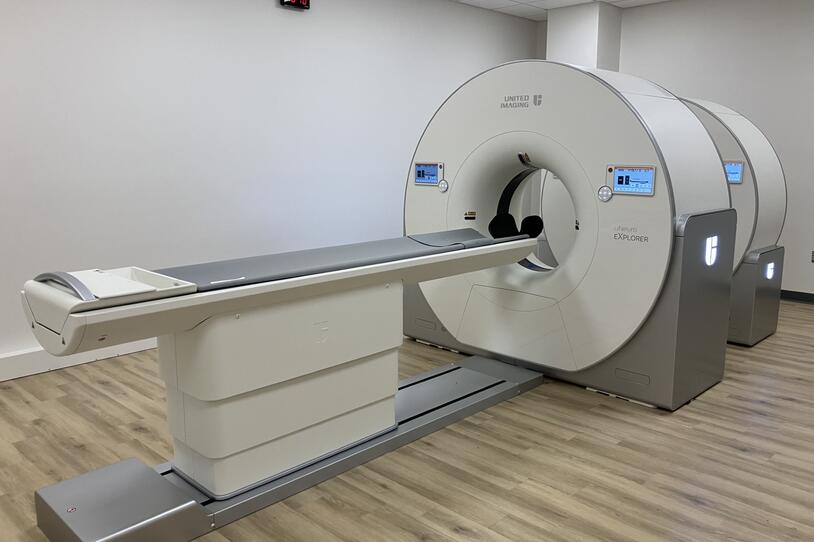
In addition, a new camera in New Haven, Connecticut called the Neuroexplorer could help.
Xing Imaging recently installed a Neuroexplorer PET imaging camera at their facility in Connecticut — the fourth such camera in the world. Xing serves as the imaging core for MJFF’s flagship Parkinson’s Progression Markers Initiative (PPMI) providing imaging support when needed, and now, their imaging capabilities are expanded in important ways.
This new PET camera has a dramatically higher resolution and sensitivity than other commonly used PET cameras, meaning we get a much clearer image of what tracers are highlighting. We are also able to see much smaller parts of the brain, like the substantia nigra, a part of the brain that plays a big role in neurodegenerative diseases.
Dr. Eberling explains, “Think of it sort of like the improvement of cameras on your iPhone. Look how much better they are since the iPhone came out in 2007.”
The new camera will improve the output of all the tracers in development. “Whatever advances you make in this technology should apply to any PET tracer you’re looking at,” says Dr. Eberling. “Good or bad — the camera should improve the quality of image we’re looking at when trying to assess progress developing tracers. This is especially true for smaller brain structures that have traditionally been harder to capture.”
“For therapeutic development, the biggest thing we need right now are good biomarkers,” Dr. Eberling adds. Biomarkers are a big part of the MJFF research portfolio, and the Foundation validated the first biomarker for Parkinson’s (the alpha-synuclein seeding amplification assay) in 2023. However, that biomarker is not quantitative, meaning it detects if alpha-synuclein is clumping, but it cannot show change over time—a key component of any biomarker for use in clinical trials. Imaging has the potential to provide that kind of tracking.
“There could be therapies out there that work, but we’re just not able to measure them well enough to know that they work. More accurate imaging is one way to support the trials that test these treatments, making them faster and more efficient,” Eberling concludes.
MJFF continues to pursue biomarkers of every sort—from biofluids to imaging—because as our technology improves, so too does our ability to address the underlying biology of Parkinson’s disease.